中文同义词:
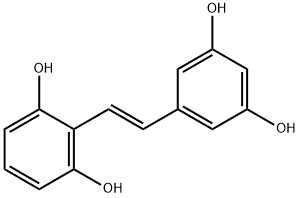
2,6,3,5-四羟基二苯乙烯;买麻藤醇
英文名称:
2-[(1E)-2-(3,5-Dihydroxyphenyl)ethenyl]-1,3-benzenediol
英文同义词:
2-[2-(3,5-dihydroxyphenyl)ethenyl]benzene-1,3-diol;2-[(1E)-2-(3,5-Dihydroxyphenyl)ethenyl]-1,3-benzenediol;Gnetol;2,3,5,6-Tetrahydroxy-trans-stilbene;-2-(3,5-Dihydroxystyryl);Gnetol>;(E)-2-(3,5-Dihydroxystyryl)benzene-1,3-diol;1,3-Benzenediol, 2-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]-
相关类别:
植物提取物;醇;中药对照品;其它酚类;标准品 -中药标准品;对照品;标准品-对照品;标准品,对照品
沸点
540.8±30.0 °C(Predicted)
密度
1.468±0.06 g/cm3(Predicted)
储存条件
Inert atmosphere,2-8°C
酸度系数(pKa)
9.06±0.40(Predicted)
最大波长(λmax)
337nm(EtOH)(lit.)
简介
2,6,3,5-四羟基二苯乙烯又叫买麻藤醇。买麻藤醇是从买麻藤属植物中分离得到的一个二苯乙烯单体化合物, 药理实验表明, 它具有较强的抗炎和抗氧化活性。
应用
2,6,3,5-四羟基二苯乙烯可用于制备4-(6,8-二甲氧基-2-萘基)-1,3-苯二醇。2,6,3,5-四羟基二苯乙烯为新型的2,6,3,5-四羟基二苯乙烯二聚体失去两个苯环生成的苯基萘衍生物,药理活性测试结果表明, 4-(6,8-二甲氧基-2-萘基)-1,3-苯二醇显示有较强的抗氧化活性。
提取方法
小叶买麻藤藤茎10 kg, 80 %乙醇热提 3 次, 减压回收溶剂得乙醇提取物, 用热水分散后依次用石油醚、乙酸乙酯、正丁醇萃取。用多种柱层析和薄层制备层析等方法, 从乙酸乙酯部位分得化合物 Ⅰ(20 mg) 、Ⅱ(30 mg) 、Ⅲ(8 mg) ;Ⅳ (15 mg) 、Ⅴ(20 mg) 、Ⅵ (20 mg ) 、Ⅶ (400 mg) 、Ⅷ (100 mg) 、Ⅸ(33 mg) , 从正丁醇部位分得化合物Ⅹ (150 mg) 。Ⅸ即2,6,3,5-四羟基二苯乙烯。
生物活性
Gnetol 是从 Gnetum ula Brongn 的根中分离出来的酚类化合物。Gnetol 有效抑制 COX-1 (IC50 为 0.78 μM) 和 HDAC。Gnetol 是一种有效的酪氨酸酶抑制剂,对鼠酪氨酸酶的 IC50 为 4.5 μM,可抑制黑色素的生物合成。Gnetol 具有抗氧化,抗增殖,抗癌和保肝活性。Gnetol 还具有浓度依赖性的 α-淀粉酶,α-葡萄糖苷酶和脂肪形成活性。
靶点
COX-1
0.78 μM (IC 50 )
Tyrosinase
4.5 μM (IC 50 )
HDAC
COX-1
0.78 μM (IC 50 )
Tyrosinase
4.5 μM (IC 50 )
体外研究
The antiproliferative activities of Gnetol are tested in HCT-116, Hep-G2, MDA-MB-231, and PC-3 cell lines by measuring cell viability after treatment with 4.1 μM, 40.9 μM, 204.7 μM, 409.4 μM, and 1023.6 μM. Gnetol shows concentration-dependent reductions in cell viability in cancer cell lines with greatest activity in colorectal cancer.
Gnetol at 200 µg/mL significantly offers the highest protection of 54.3% against the toxicant. A lower dose of Gnetol (50 µg/mL) also shields the cell line from the toxic effects of CCl4.
The ligand molecule TGF-β and PPARα protein show that Gnetol has the binding affinity of 7.0 and 8.4, respectively.
体内研究
Male Sprague-Dawley rats were cannulated and dosed either intravenously with Gnetol (10 μg/kg) or orally (100 mg/kg). After oral and intravenous administration, Gnetol is detected in both serum and urine as the parent compound and as a glucuronidated metabolite. The bioavailability of Gnetol is determined to be 6%. Gnetol is rapidly glucuronidated and is excreted in urine and via nonrenal routes.
Pretreatment of Male NIH Swiss mice (20-35 g) with Gnetol (50mg/kg, SC) is able to increase the latency period to response in analgesia models.
 15623309010
15623309010






