英文名:4-(2-(5-Chloro-2-methoxybenzamido)ethyl)benzenesul
外观:
纯度:请咨询卖家
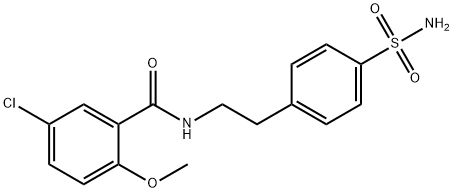
分子式:C16H17ClN2O4S
分子量:368.84
最小起售量:10g/20g/100g/1kg
中文名称:
4-[2-(5-氯-2-甲氧基苯甲酰氨基)乙基]苯磺酰胺
中文同义词:
4-[2-(2-甲氧基-5-氯苯甲酰胺基)乙基]苯磺酰胺;5-氯-2-甲氧基-N-[2-对氨磺酰苯基)乙基]苯甲酰氨;N-{2-[4-(氨磺酰基)苯基]乙基}-5-氯-2-甲氧基苯甲酰胺;格列本脲中间体;格列本脲相关物质A;格列本脲USP RC A;格列本脲(优降糖)杂质A;4-[2-(5-氯-2-甲氧基-苯甲酰氨基)乙基]苯磺酰胺
英文名称:
4-(2-(5-Chloro-2-methoxybenzamido)ethyl)benzenesulfamide
英文同义词:
4-(2-(5-Chloro-2-methoxybenzamido)ethyl)benzenesulfamide;Glyburide Related Compound A (25 mg) (5-chloro-2-methoxy-N-[2-(4-sulfamoylphenyl)ethyl]benzamide);4-[2-(2-Methoxy-5-chlorobenzene-1-carboxaMido)ethyl]benzenesulfonaMide;5-Chloro-N-(p-sulfaMoylphenethyl)-o-anisaMide;5-Chloro-2-Methoxy-N-(4-sulfaMoylphenethyl)benzaMide;GlibenclaMide (Glyburide) IMpurity A;Glyburide Related CoMpound A;Glyburide Related Compound A (25 mg) (4-[2-(5-chloro-2-methoxybenzamido)ethyl]benzenesulfonamide)
相关类别:
磺胺类药物/亚磺酰胺;磺胺类药物;亚磺酰胺;中间体;有机化学;Pharmaceuticals;Organic Building Blocks;Sulfonamides/Sulfinamides;Sulfur Compounds;Aromatics;Intermediates Fine Chemicals;Aromatics,Pharmaceuticals;Building Blocks;Chemical Synthesis;Organic Building Blocks;Sulfur Compounds
密度
1.356±0.06 g/cm3(Predicted)
酸度系数(pKa)
10.14±0.10(Predicted)
CAS 数据库
16673-34-0(CAS DataBase Reference)
生物活性
NLRP3 Inflammasome Inhibitor I是合成格列本脲的中间底物,它是NLRP3 inflammasome的抑制剂。
靶点
Target Value
NLRP3 inflammasome
()
体内研究
NLRP3-IN-2 is well tolerated with no effects on the glucose levels in vivo.
NLRP3-IN-2 (100 mg/kg) treatment in a model of AMI due to ischemia+reperfusion significantly inhibits the activity of inflammasome (caspase-1) in the heart by 90% (P<0.01) and reduced infarct size, measured at pathology (by >40%, P<0.01) and with troponin I levels (by >70%, P<0.01) .
Animal Model: Experimental acute myocardial infarction (AMI) model in mice.
Dosage: 100 mg/kg.
Administration: Intraperitoneal administration 30 minutes prior to surgery, then every 6 hours for 3 additional doses.
Result: Led to a significant >90% reduction in caspase-1 activity (reflective of the formation of an active inflammasome) in the heart tissue measured 24 hours after ischemia.
Led to a significant reduction in the infarct size measured with TTC (>40% reduction) or troponin I levels (>70% reduction) when compared with vehicle alone.
Animal Model:
Experimental acute myocardial infarction (AMI) model in mice.
Administration:
Intraperitoneal administration 30 minutes prior to surgery, then every 6 hours for 3 additional doses.
Result:
Led to a significant >90% reduction in caspase-1 activity (reflective of the formation of an active inflammasome) in the heart tissue measured 24 hours after ischemia.
Led to a significant reduction in the infarct size measured with TTC (>40% reduction) or troponin I levels (>70% reduction) when compared with vehicle alone.
生产方法
用水杨酸在光照下以氯气氯化,生成5-氯水杨酸,然后以硫酸二甲酯甲基化得5-氯-2-甲氧基苯甲酸,将后者以氯化亚硫酰氯化,得5-氯-2-甲氧基苯甲酰氯,再与苯胺缩合,产物为N-苯乙基-5-氯-2-甲氧基苯甲酰胺,进而以氯磺酸氯磺化、氨水胺化制得该品。
 15623309010
15623309010






