中文同义词:
纳呋拉啡盐酸;盐酸纳呋拉啡;盐酸呐呋拉菲;纳呋拉啡盐酸盐
英文同义词:
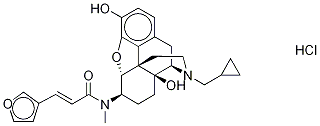
TRK-820 HYDROCHLORIDE;(E)-N-((4R,4aS,7R,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-yl)-3-(furan-3-yl)-N-methylacrylamide;17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-epoxy-6beta-[N-methyl-trans-3-(3-furyl)acrylamido]morphinan hydrochloride;2-Propenamide, N-[(5a,6b)-17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-yl]-3-(3-furanyl)-N-methyl-,hydrochloride (1:1), (2E)-
相关类别:
医药原料药;小分子抑制剂;小分子抑制剂,天然产物;医药原料;Agonists;Chiral Reagents;Heterocycles;Intermediates Fine Chemicals;Neurochemicals;Pharmaceuticals;Inhibitors
生物活性
Nalfurafine hydrochloride (TRK-820 hydrochloride) 是一种强效选择性,具有口服活性的 G 蛋白偏倚 kappa opioid receptor (KOR) 激动剂,具有很高的翻译潜力。Nalfurafine hydrochloride (TRK-820 hydrochloride) 增强了 MOR 靶向镇痛药的生物学作用,它还有潜力用于尿毒症瘙痒的相关研究。
体内研究
Nalfurafine (subcutaneous injection; 0.015 mg/kg) combines with EOM-salvinorin-B produces spinal antinociception equivalent to 5 mg/kg, it also enhances the supraspinal analgesic effect of 5 mg/kg morphine.Nalfurafine (subcutaneous injection; 4 μg/kg) causes a dose-dependent increase of the inhibition of the acetic acid-induced abdominal constriction,and the inhibition of the abdominal constriction reaches its peak 30 min after injection, gradually declined and returned to the pre-injection level 4 hr after.
Animal Model: Male and Female C57BL/6J mice
Dosage: 0.015 mg/kg
Administration: Subcutaneous injection
Result: Had the potential for enhancing the therapeutic potential of MOR-targeting analgesics, such as morphine.
Animal Model:
Male and Female C57BL/6J mice
Administration:
Subcutaneous injection
Result:
Had the potential for enhancing the therapeutic potential of MOR-targeting analgesics, such as morphine.
用途
Nalfurafine hydrochloride was launched on March of 2009 in Japan as the first in class non-narcotic opioid drug for intractable itch caused by hemodialysis. It showed significant opioid 魏-agonist acti vity and induced neither aversion nor preference in rats on the CPP (Conditioned Place Preference) test. A new therapeutic agent for the treatment of uremic pruritus in hemodialysis patients.
 15623309010
15623309010






