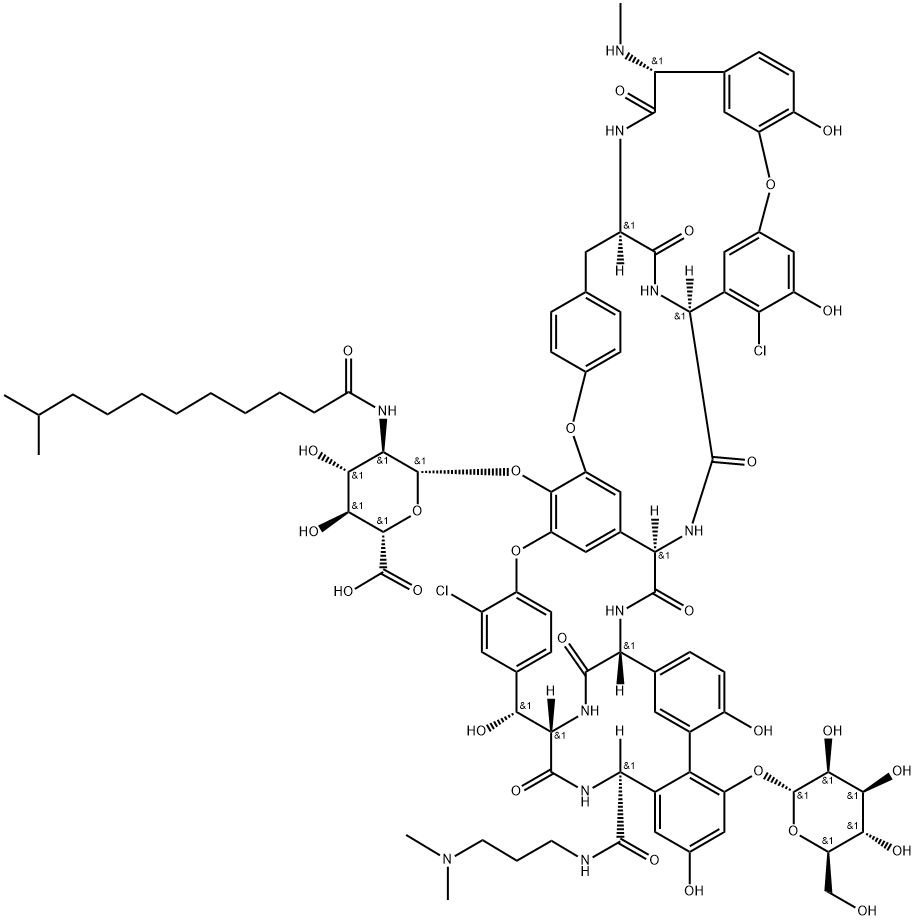
达巴万星
中文名称:
达巴万星
中文同义词:
达巴万星 道古霉素;道古霉素盐酸盐包装;道古霉素盐酸盐多少钱;DALBAVANCIN达巴万星;道古霉素盐酸盐;道古霉素(达巴万星);达巴霉素;道古霉素,达巴万星,达巴霉素
英文名称:
Dalbavancin
英文同义词:
Dalbavancin;CS-2250;Dalbavancin Ao;Dalbavancin(BI 397;DALBAVANCIN;MDL 63397;A-A-1; DALBAVANCIN AO; BI 397; MDL 63397;DALBAVANCIN(BI 397; MDL 63397);Dalbavacin;Antibotic Dalbavancin;DB/ QTH04
CAS号:
171500-79-1
分子式:
C88H100Cl2N10O28
分子量:
1816.71
EINECS号:
相关类别:
微生物代谢物;达巴万星原料 171500-79-1;医药原料药;医药化工类;科研试剂;医药原料;对照品-中药对照品;原料药;主打产品
Mol文件:
171500-79-1.mol
密度
1.59
储存条件
-20°C
形态
powder
酸度系数(pKa)
2.73±0.70(Predicted)
颜色
white to beige
达巴万星
达巴万星(Dalbavancin),也称作道古霉素,它是一种新型半合成糖肽抗生素,为替考拉宁类似物A40926的衍生物,由美国Vicuron Pharmaceuticals公司发现,开发并进入临床实验,后来此公司被辉瑞公司收购。
达巴万星作用机制与万古霉素和替考拉宁相同,抑制G+菌细胞壁的生物合成,被广泛用作治疗皮肤和软组织感染的药物。体内、外试验表明,达巴万星对于G+菌包括耐甲氧西林金葡球菌(MRSA)、甲氧西林敏感金葡球菌(MSSA)、凝固酶阴性葡萄球菌(CoNS)、链球菌等具有抗菌活性。对耐G+病原菌包括耐青霉素和头孢曲松肺炎链球菌、替考拉宁不敏感CoNS、非vanA型肠球菌具有活性;对G+厌氧菌也具有活性。达巴万星具有独特的药动学性质,可每周间隔用药。目前,达巴万星在治疗导管相关的血源性感染以及皮肤和软组织感染已取得了良好的效果,其具有优良的体内抗菌活性和安全性,是理想的第二代糖肽抗生素。
生物活性
Dalbavancin (Zeven, BI 397, MDL 63397)是一种脂糖肽类抗生素,对革兰氏阳性菌,如多种葡萄状球菌,具有杀菌活性。
靶点
MIC90: 0.06 μg/mL ( Staphylococcus aureus ) and 0.25 μg/mL ( Bacillus anthracis )
体外研究
Dalbavancin is a parenterally administered semisynthetic lipoglycopeptide developed to combat infections caused by resistant gram-positive pathogens. Dalbavancin exhibits potent in vitro bactericidal activity against gram-positive pathogens including S. aureus (MRSA), VISA, and non-VanA strains of VRE. Dalbavancin is developed for the treatment of complicated skin and skin structure infections (cSSSIs), predominantly those caused by MRSA and β-hemolytic streptococci , organisms against which it has shown greater potency than existing glycopeptide therapeutic agents.
体内研究
Dalbavancin (15-240 mg/kg; intraperitoneal injection; every 36 h or 72 h; for 14 days; female BALB/c mice) treatment has a survival rate of 80% to 100% of mice with all dose regimens.
Animal Model: Female BALB/c mice (6-8 weeks) challenged with Ames strain of B. anthracis
Dosage: 15 mg/kg, 30 mg/kg, 60 mg/kg, 120 mg/kg, 240 mg/kg
Administration: Intraperitoneal injection; every 36 h or 72 h; for 14 days
Result: The efficacy was 80 to 100%, as determined by the rate of survival at 42 days, when treatment was initiated 24 h postchallenge with regimens of 15 to 120 mg/kg every 36 h or 30 to 240 mg/kg every 72 h.
Animal Model:
Female BALB/c mice (6-8 weeks) challenged with Ames strain of B. anthracis
Dosage:
15 mg/kg, 30 mg/kg, 60 mg/kg, 120 mg/kg, 240 mg/kg
Administration:
Intraperitoneal injection; every 36 h or 72 h; for 14 days
Result:
The efficacy was 80 to 100%, as determined by the rate of survival at 42 days, when treatment was initiated 24 h postchallenge with regimens of 15 to 120 mg/kg every 36 h or 30 to 240 mg/kg every 72 h.








