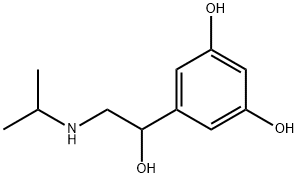
奥西那林
中文名称:
奥西那林
中文同义词:
奥西那林;5-(1-羟基-2-异丙基氨基乙基)苯-1,3-二酚;间羟异丙基肾上腺素
英文名称:
Orciprenaline
英文同义词:
5-[1-hydroxy-2-(1-methylethylamino)ethyl]benzene-1,3-diol;5-(1-hydroxy-2-propan-2-ylamino-ethyl)benzene-1,3-diol;orciprenaline;1-(3,5-dihydroxyphenyl)-2-isopropylaminoethanol;1,3-Benzenediol, 5-1-hydroxy-2-(1-methylethyl)aminoethyl-;1,3-Benzenediol, 5-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]- (9CI);3,5-Dihydroxy-a-[(isopropylamino)methyl]benzyl alcohol;Benzyl alcohol, 3,5-dihydroxy-a-[(isopropylamino)methyl]- (6CI, 8CI)
CAS号:
586-06-1
分子式:
C11H17NO3
分子量:
211.26
EINECS号:
209-569-1
相关类别:
瘦肉精类;平喘药物;Orciprenaline
Mol文件:
586-06-1.mol
熔点
100°
沸点
350.94°C (rough estimate)
密度
1.1240 (rough estimate)
折射率
1.5718 (estimate)
酸度系数(pKa)
9.11±0.10(Predicted)
CAS 数据库
586-06-1(CAS DataBase Reference)
生物活性
Metaproterenol (Orciprenaline) 是一种直接作用的拟交感神经药,是 β2-肾上腺素能受体 (β2AR) 激动剂,其 IC50 为 68 nM。Metaproterenol 还具有抗炎活性。
靶点
IC50: 68 nM (β2-adrenergic receptor)
体外研究
Metaproterenol (10 μM; 74 hours; THP-1 cells and bone marrow macrophages) treatment enhances β-arrestin2 and its interaction with IκBα in high glucose-induced THP-1 cells and bone marrow macrophages.
Metaproterenol (10 μM; 74 hours; THP-1 cells and bone marrow macrophages) treatment leads to downregulation of NF-κB in high glucose-induced THP-1 cells and bone marrow macrophages.
Western Blot Analysis
Cell Line: THP-1 cells and bone marrow macrophages
Concentration: 10 μM
Incubation Time: 74 hours
Result: Enhanced β-arrestin2 and its interaction with IκBα.
RT-PCR
Cell Line: THP-1 cells and bone marrow macrophages
Concentration: 10 μM
Incubation Time: 74 hours
Result: Led to downregulation of NF-κB.
Cell Line:
THP-1 cells and bone marrow macrophages
Concentration:
10 μM
Incubation Time:
74 hours
Result:
Enhanced β-arrestin2 and its interaction with IκBα.
Cell Line:
THP-1 cells and bone marrow macrophages
Concentration:
10 μM
Incubation Time:
74 hours
Result:
Led to downregulation of NF-κB.
体内研究
Treatment of Zucker diabetic fatty rats with Metaproterenol for 12 weeks attenuates monocyte activation as well as pro-inflammatory and pro-fibrotic responses in the kidneys and heart. Thus, Metaproterenol might has protective effects against diabetic renal and cardiovascular complications.
化学性质
熔点100℃。其硫酸([5874-97-5])熔点202-203℃。
用途
支气管扩张药。
生产方法
3,5-二乙酸基乙酰首先与溴反应,然后与异丙胺反应得过且过-(3,5-二羟基苯基)-2-异丙胺乙酮,将其溶于甲醇,在室温下以镍催化氢化,得异丙喘宁。








