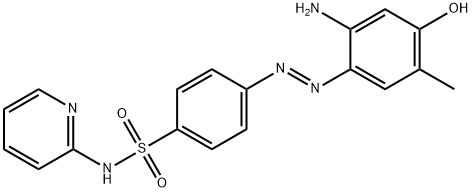
中文名称:
(E)-4-((2-AMINO-4-HYDROXY-5-METHYLPHENYL)DIAZENYL)-N-(PYRIDIN-2-YL)BENZENESULFONAMIDE
中文同义词:
4-[(1E)-2-(2-氨基-4-羟基-5-甲基苯基)偶氮]-N-2-吡啶基苯磺酰胺;BROMODOMAIN抑制剂(MS436)
英文同义词:
(E)-4-[2-(2-Amino-4-hydroxy-5-methylphenyl)diazenyl]-N-2-pyridinylbenzenesulfonamide;MS-436; MS 436;4-[(2Z)-2-(2-amino-5-methyl-4-oxocyclohexa-2,5-dien-1-ylidene)hydrazinyl]-N-pyridin-2-ylbenzenesulfonamide;CS-1882;MS436(95%);4-[(1E)-2-(2-Amino-4-hydroxy-5-methylphenyl)diazenyl]-N-2-pyridinylbenzenesulfonamide;4-[(1E)-2-(2-Amino-4-hydroxy-5-methylphenyl)diazenyl]-N-2-pyridinylbenzenesulfonamide MS436;MS436 4-[(1E)-2-(2-Amino-4-hydroxy-5-methylphenyl)diazenyl]-N-2-pyridinylbenzenesulfonamide
相关类别:
小分子抑制剂;小分子抑制剂,天然产物;细胞生物学试剂;Inhibitors
沸点
673.7±65.0 °C(Predicted)
密度
1.42±0.1 g/cm3(Predicted)
酸度系数(pKa)
9.25±0.36(Predicted)
生物活性
MS436 是一种 bromodomain 抑制剂,有效作用于 BRD4 BrD1,Ki 为 30-50 nM,比作用于 BrD2 选择性高 10 倍。
靶点
Ki: 30-50 nM (BRD4 bromodomain)
体外研究
MS436, through a set of water-mediated interactions, exhibits low nanomolar affinity (estimated K i of 30-50 nM) with preference for the first bromodomain over the second. MS436 effectively inhibits BRD4 activity in NF-κB-directed production of NO and pro-inflammatory cytokine interleukin-6 in murine macrophages. MS436 represents a new class of bromodomain inhibitors and will facilitate further investigation of the biological functions of the two bromodomains of BRD4 in gene expression. MS436 exhibits potent affinity of an estimated K i =30-50 nM for the BRD4 BrD1 and a 10-fold selectivity over the BrD2, which is achieved through a unique set of water-mediated intermolecular interactions.